1 min to read
Protein fibrils formed with phosphoric acid
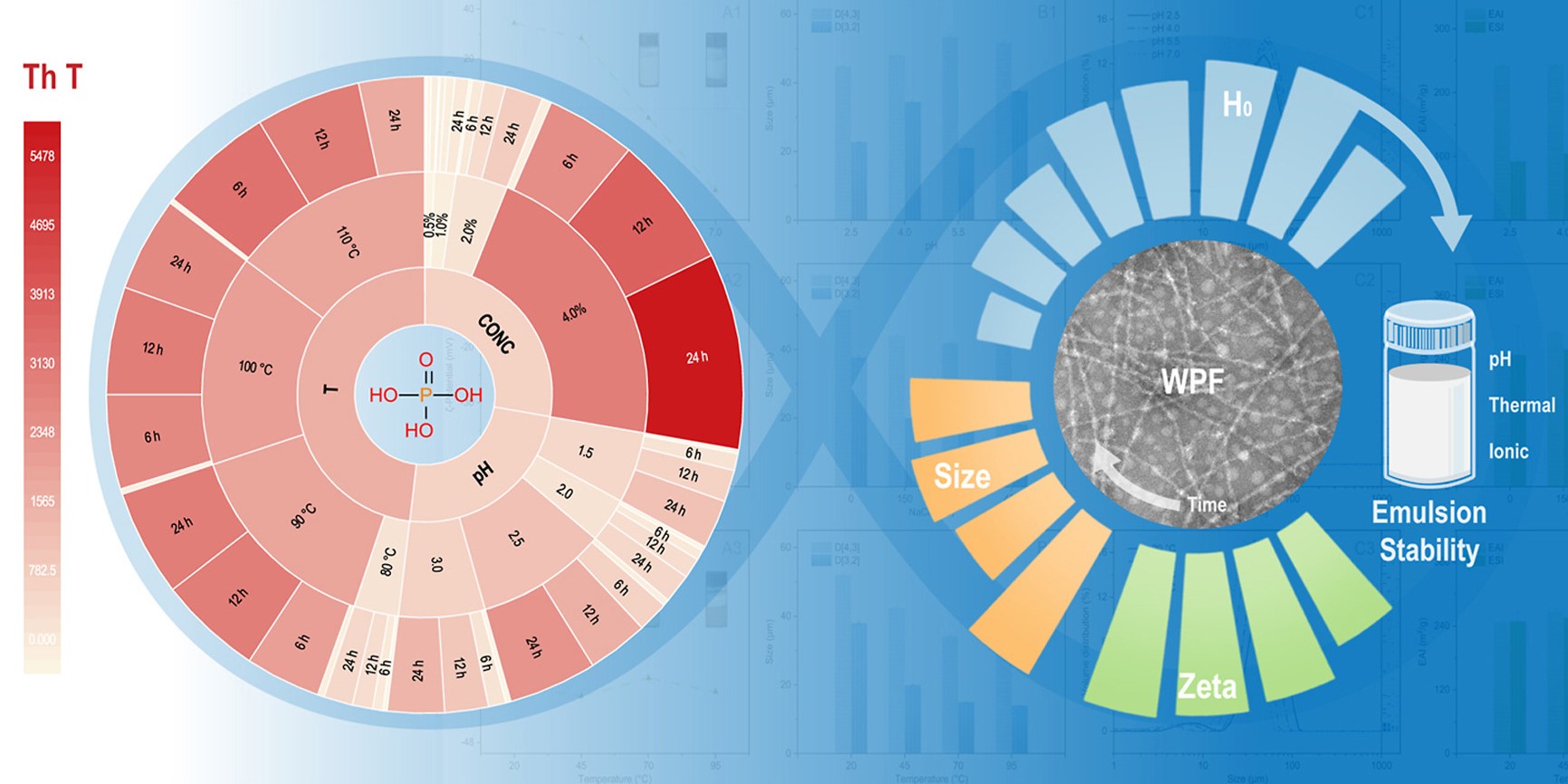
Formation, structural characteristics, and emulsion stability.
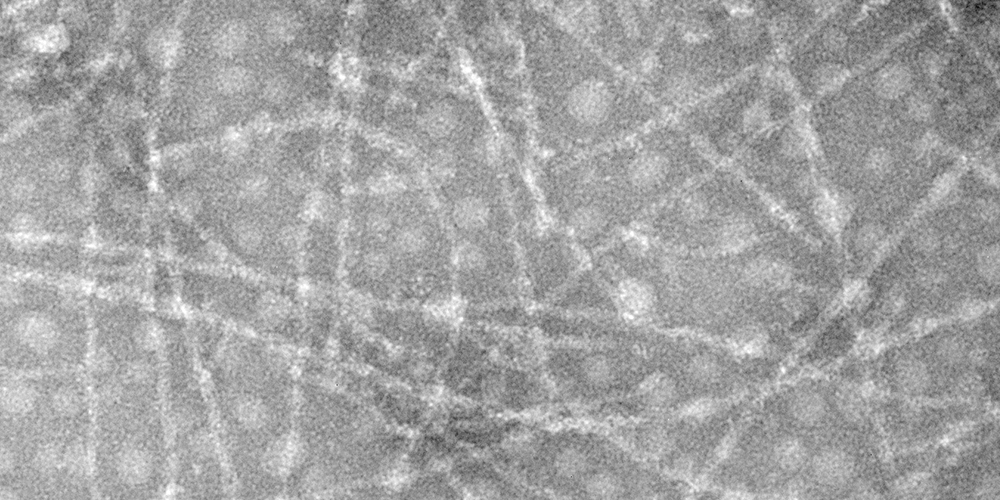
In this study, phosphoric acid was used to replace hydrochloric acid which is widely reported in the production of whey protein isolate fibrils (WPF). The pH, temperature, and protein concentration for the phosphoric acid-induced fibrillation were optimized (4% w/v, pH 2.5, 90 °C). Under this condition, the mean particle size and molecular weight decreased significantly while the ζ-potential increased slightly, it suggested that the fibrillation was accompanied by hydrolysis. WPF was already formed via intermolecular interactions at 6 h, which exhibited a semiflexible and unbranched filament of about 10 nm in diameter. A few single-filament flexible fibrils (about 5 nm in diameter) formed at 24 h. Thioflavin T analysis supported by Intrinsic fluorescence showed that the use of phosphoric acid accelerated the fibrillation. The ANS fluorescence and Second-derivative UV–vis spectra indicated the increase in surface hydrophobicity, peaking after 12 h of heating. Compared with the hydrochloric acid induced, WPF induced by phosphoric acid exhibited a shorter lag phase and maintained a more stable dispersion state with a nanoscale particle size. The great interfacial properties and proper aspect ratio (length/diameter) provided the contribution that WPF effectively absorbed at the oil-water interface. Moreover, the emulsion stability of the WPF improved with increasing salt concentration. The phosphoric acid-induced WPF had the best emulsion stability at pH 5.5 which was further enhanced by proper heating.
Graphical Abstract
Find the full text
The full text can be found on the Food Hydrocolloids 2023 Vol. 135 Pages 108170.
Thank you for careful reading.

Comments